Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kawaguchi, Munemichi
Proceedings of 2020 International Conference on Nuclear Engineering (ICONE 2020) (Internet), 6 Pages, 2020/08
In decommissioning sodium-cooled fast reactors, the operators can be exposed to radiation during dismantling of the cold trap equipment (C/T). The C/T has higher dose equipment because the C/T trapped the tritium of the fission product during the operation to purify the sodium coolant. In this research, the thermal decomposition temperature and rate of sodium hydride were measured as the fundamental research for improvement of the thermorlysis method prior to the dismantling. We measured the thermal decomposition temperature and rate using sodium hydride powder (95.3%, Sigma-Aldrich) in Al O
O crucible with TG-DTA (STA2500, NETASCH Japan). The heating rates were set to
crucible with TG-DTA (STA2500, NETASCH Japan). The heating rates were set to  = 2.0, 5.0, 10.0, 20.0 K/min, and the weight decrease was measured. The thermal decomposition temperature and rate were obtained from the temperature of the onset of the weigh decrease and the Kissinger plot, respectively. Furthermore, we set to the thermal decomposition temperature of around 600 K, and the weight decreasing rates were measured. The change of the chemical composition of the sodium hydride with heating (from NaH to Na) was measured with X-Ray Diffraction (XRD) analysis. As a result, the thermal decomposition occurred at around 600 K, and the almost all hydrogen in the sodium hydride released within 1 h. The thermal decomposition rate strongly depended on the heating temperature.
= 2.0, 5.0, 10.0, 20.0 K/min, and the weight decrease was measured. The thermal decomposition temperature and rate were obtained from the temperature of the onset of the weigh decrease and the Kissinger plot, respectively. Furthermore, we set to the thermal decomposition temperature of around 600 K, and the weight decreasing rates were measured. The change of the chemical composition of the sodium hydride with heating (from NaH to Na) was measured with X-Ray Diffraction (XRD) analysis. As a result, the thermal decomposition occurred at around 600 K, and the almost all hydrogen in the sodium hydride released within 1 h. The thermal decomposition rate strongly depended on the heating temperature.
Kawaguchi, Munemichi
no journal, ,
In decommissioning sodium-cooled fast reactors, the thermal decomposition behavior of tritium is important from the viewpoint of reducing the exposed radiation for the operators during dismantling of the cold trap equipment. In this study, the thermal decomposition temperature and rate of sodium hydride (95.3%, Sigma-Aldrich) were estimated from the TG-DTA measurements. The change of the chemical composition for the thermal decomposition was measured with X-Ray Diffraction analysis.
Kawaguchi, Munemichi
no journal, ,
This study presents the fundamental research on the thermal decomposition behavior of sodium hydride (NaH) in FY2021. This study on the thermal decomposition behavior of NaH was carried out using TG-DTA and FT-IR for the decommissioning of the cold trap in Monju. Due to the weak ionic bonding of Na-H in NaH, the hydrogen is enable to move easily at close to 565K. Upon further heating, the hydrogen desorbs by the thermal decomposition. A simple model using each kinetic rate equations (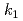 ,
, 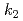 ,
, 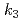 ) can give us the quantitative evaluation of the thermal decomposition behavior of NaH, which tended to overestimated the Na evaporation.
) can give us the quantitative evaluation of the thermal decomposition behavior of NaH, which tended to overestimated the Na evaporation.